Gut Microbiome Changes and ASCA and Anti-GP2 Antibodies in IBD: Evidence from a Large Family Cohort
Inflammatory bowel diseases (IBD), which include Crohn’s disease (CD) and ulcerative colitis (UC), are chronic disorders driven by immune dysregulation, environmental triggers, genetic predisposition, and alterations in the gut microbiota. Growing evidence highlights the role of serological biomarkers in IBD in improving disease characterization, early diagnostic refinement, and patient stratification. A new large-scale prospective investigation (state 2025)—the KINDRED IBD Family Cohort Study, initiated in 2013 —provides critical insights into how the gut microbiota evolves in IBD patients and their high-risk relatives1. Its findings help clarify the biological mechanisms behind IBD and point toward early diagnostic and serological markers and microbial signatures associated with disease severity.
What the Study Investigated
Researchers collected extensive data of a cohort with more than 2,300 individuals, including IBD patients and their first-degree relatives on:
- 16S rRNA microbiome sequencing
- Clinical and nutritional data
- Genetic profiles (polygenic risk scores)
- Inflammatory biomarkers such as calprotectin, CRP, ASCA2, and anti-GP23 antibodies
This design enabled a detailed analysis of microbial disruption, genetic susceptibility, and early signs of disease onset.
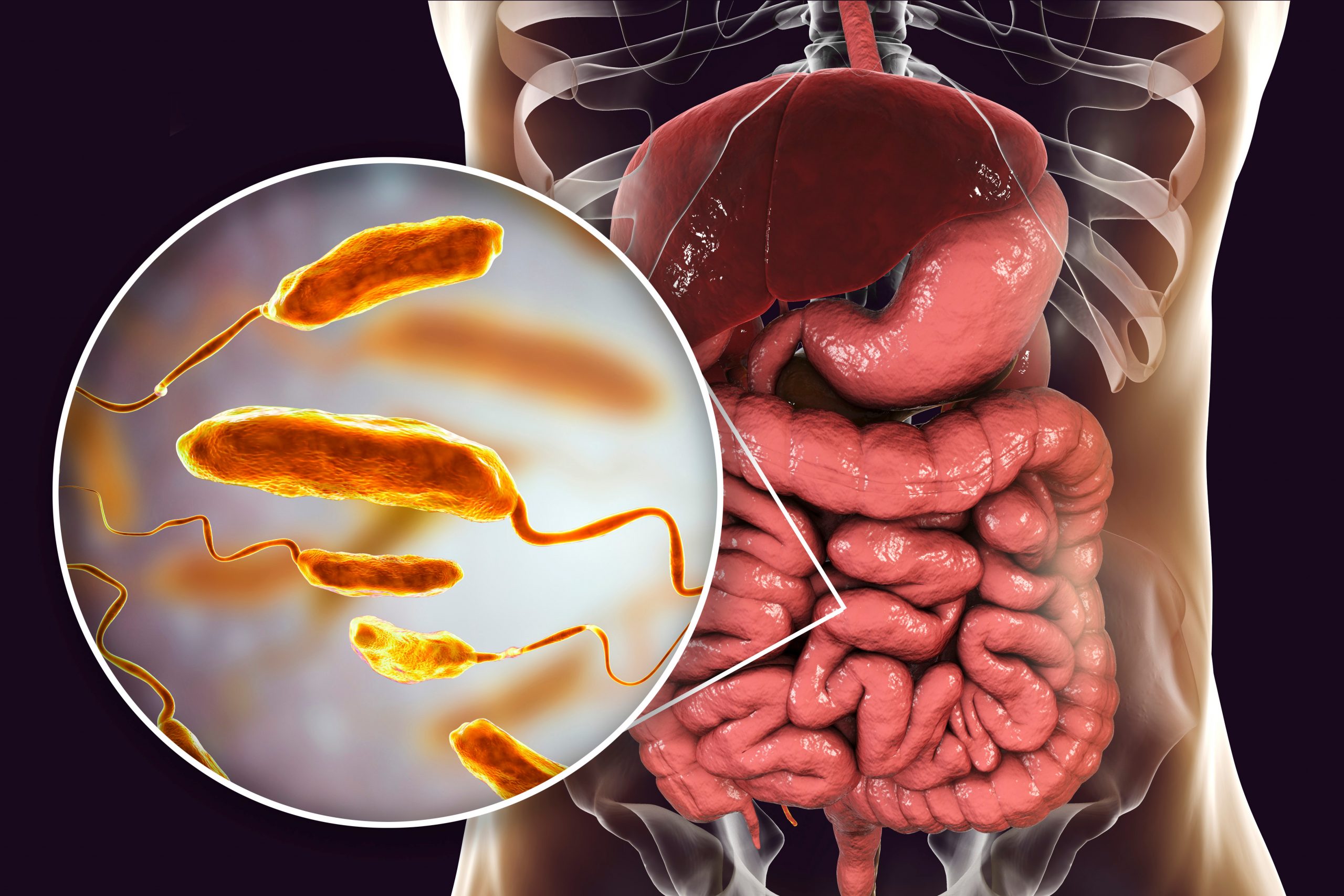
Major Gut Microbiome Alterations in Crohn’s Disease and Ulcerative Colitis
An essential discovery was the pronounced microbial dysbiosis —disruption or alterations in the composition, function, or stability of the microbial communities in gut—in individuals with CD and UC. The key characteristics included:
1. Significant Loss of Microbial Diversity
Both CD and UC patients showed reduced microbial diversity, especially in Crohn’s disease. Lower diversity correlated with higher fecal calprotectin, elevated ASCA IgA/IgG levels, and softer stools, as well as faster transit time.
These relationships confirm that microbiome instability mirrors intestinal inflammation.
2. Expansion of Harmful Bacteria (Enterobacteriaceae)
Patients with IBD consistently showed increased levels of e.g., Klebsiella, Escherichia/Shigella, and Raoultella.
These members of the Enterobacteriaceae family are inflammation-associated and prosper in disturbed gut ecosystems.
3. Oral-to-gut microbial translocation
The presence of oral-origin bacteria, including Fusobacterium nucleatum, Veillonella, Rothia, and Candidatus Saccharibacteria, in the intestines of IBD patients was a remarkable finding.
These microbes are rarely abundant in healthy gut communities and indicate a loss of resistance to colonization.
4. Reduction of Beneficial Bacteria
Healthy subjects showed higher levels of Faecalibacterium, Ruminococcaceae, Alistipes, and Prevotella compared to IBD patients.
These bacteria support gut barrier function and anti-inflammatory pathways.
Genetic Risk and Microbiome Profiles
The study also linked polygenic risk for IBD to specific microbial patterns. Individuals with higher genetic risk showed:
- Increased ASCA and anti-GP2 antibodies
- Greater microbiota alteration
- More inflammation-associated bacteria
This demonstrates that genetics and microbiome composition interact tightly in IBD development.
Insights from Individuals Who Later Developed IBD
One unique value of this study was its ability to monitor individuals who progressed from health to IBD. These individuals did not show severe microbiota alteration before diagnosis, suggesting that profound microbial disruption may occur after clinical onset. Nevertheless, they exhibited subtle shifts in specific bacterial groups and inflammatory markers.
ASCA and Anti-GP2 Antibodies as Serological Biomarkers in IBD
The study confirmed that ASCA (IgA/IgG) and anti-GP2 (IgA/IgG) antibodies are highly informative serological biomarkers in IBD, especially in Crohn’s disease. ASCA levels were significantly elevated in CD patients and strongly associated with Enterobacteriaceae overgrowth, increased calprotectin, and signs of accelerated gut transit. Anti-GP2 antibodies also increased—particularly in CD—and correlated with age, sex, microbial dysbiosis, and polygenic IBD risk.
Taking into consideration the routine clinical laboratory workflow, performing ASCA and anti-GP2 ELISA assays offers several operational and clinical advantages compared with the broader set of diagnostic tools discussed in the study. Unlike microbiome sequencing or genetic testing, ASCA and anti-GP2 ELISA assays are standardized, affordable, and easily implemented in routine diagnostic laboratories. The strong association of these serological biomarkers with Crohn’s disease and their correlation with inflammation, dysbiosis, and genetic risk factors make them practical, cost-effective biomarkers for routine patient stratification, early diagnostic refinement, and ongoing disease monitoring.
Product used in the study
| 4006 – ASCA IgA | ELISA for the quantitative determination of IgA or IgG antibodies against Saccharomyces cerevisiae (ASCA) |
| 4007 – ASCA IgG |
| 3750 – Anti-GP2 IgA | ELISA for the quantitative determination of IgA or IgG antibodies against glycoprotein 2 (GP2) |
| 3850 – Anti-GP2 IgG |