Anti–Factor H Autoantibodies and ELISA Testing: Optimizing Diagnosis in Atypical Hemolytic Uremic Syndrome (aHUS)
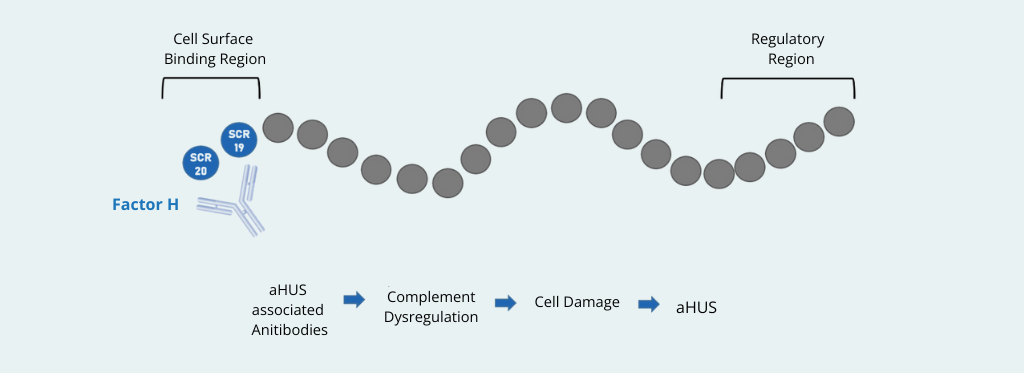
Atypical hemolytic uremic syndrome (aHUS), also known as complement-mediated thrombotic microangiopathy (CM-TMA), is a rare but life-threatening condition caused by dysregulation of the alternative complement pathway. Among the known etiologies, autoantibodies against complement factor H (anti-factor H) play a critical role, accounting for approximately 10% of cases1. Accurate laboratory detection of these antibodies is essential, as it directly impacts diagnosis, prognosis, and therapeutic strategies.
Why Anti-Factor H Antibody Testing Is Clinically Relevant
Patients with anti-factor H antibodies often benefit from targeted immunosuppressive therapies, such as corticosteroids or rituximab, in addition to plasma exchange. Early and reliable identification of anti-factor H immunoglobulin G (IgG) is therefore crucial to preventing irreversible organ damage, particularly renal failure. The ELISA assay is the reference method for detecting and quantifying anti-factor H in clinical laboratories.
However, the increasing availability of commercial kits raises important questions regarding assay comparability, sensitivity, specificity, and standardization.
Comparing ELISA Assays for Anti-Factor H Autoantibodies
A recent Canadian study compared three ELISA methods for anti-factor H antibodies detection2:

- a validated in-house ELISA based on the Paris protocol,
- a commercial ELISA from GA Generic Assays,
- and a second commercially available ELISA kit.
A total of 75 plasma samples from patients with suspected aHUS were analyzed in parallel. The study evaluated qualitative agreement, quantitative correlation, and analytical performance under routine diagnostic conditions.
Key Findings: Sensitivity, Specificity, and Concordance
All assays demonstrated good performance in identifying negative samples. However, differences emerged in positive samples and antibody quantification:
- The ELISA from GA Generic Assays showed the highest concordance with the in-house reference method. It exhibit good sensitivity, as well as strong quantitative correlation and reproducibility.
- The second commercial assay displayed greater variability, particularly at higher antibody titers, and tended to underestimate anti-factor H concentrations.
- Applying manufacturer-defined cut-offs alone increased the risk of false-negative results. However, adjusting positivity thresholds based on laboratory-specific healthy controls (e.g., mean + 2 SD) significantly improved diagnostic sensitivity with minimal loss of specificity.
These findings confirm that ELISA assays for anti-factor H antibodies are not interchangeable, especially for patient monitoring and longitudinal follow-up.
Practical Implications for Diagnostic Laboratories
The study highlights several best practices for laboratories performing anti-Factor H antibody testing:
- Use optimized cut-off values tailored to the tested population, as recommended also by the International Organization for Standardization (ISO) 15189 standard for clinical laboratory accreditation3.
- Include individual blank controls to reduce false positives caused by non-specific binding.
- When clinically relevant, confirm positive results with a second ELISA method to support treatment decisions.
- Be aware that ELISA assays detect only free circulating antibodies, potentially missing immune-complexed anti-factor H.
Conclusion

Reliable detection of anti-factor H autoantibodies is essential for modern diagnostics of atypical hemolytic uremic syndrome (aHUS). Although commercial ELISA kits provide accessible and standardized solutions, their analytical performance varies. Evidence supports using well-validated ELISA assays, such as Anti-Factor H from GA Generic Assays, in conjunction with thoughtful interpretation of results and laboratory-specific validation.
Optimizing ELISA testing for anti-factor H antibodies can lead to earlier diagnosis, more precise therapy, and improved outcomes for patients with aHUS and related complement disorders.
Product used in the study
| 4067 – Anti-Factor H | ELISA for the quantitative determination of IgG antibodies against complement factor H |
References
- Marie-Agnès Dragon-Durey et al., (2010)_Clinical features of anti-factor H autoantibody-associated hemolytic uremic syndrome ↩︎
- Thouzeau-Benghezal et al., (2025)_Comparison of 3 ELISA Assays for the Detection and Quantification of Abs anti-Complement Factor H ↩︎
- International Organization for Standardization. ISO 15189:2012 (E) Medical laboratories – Requirements for quality and competence ↩︎