ANCA dual: Two Targets, One Powerful Diagnostic Tool for ANCA-Associated Vasculitis
Anti-neutrophil cytoplasmic antibodies (ANCA)
Anti-neutrophil cytoplasmic antibodies (ANCA) are key biomarkers in the diagnosis of ANCA-associated vasculitis (AAV), a group of rare but potentially life-threatening autoimmune diseases. They can also have clinical and diagnostic value in certain non-AAV conditions. Early and accurate detection of ANCA is critical for initiating treatment promptly and improving long-term outcomes. Among the most clinically relevant targets are proteinase 3 (PR3), typically associated with granulomatosis with polyangiitis (GPA), and myeloperoxidase (MPO), typically associated with microscopic polyangiitis (MPA).
Classical indirect immunofluorescence assay (IFA) uses ethanol-fixed human granulocytes to display characteristic fluorescence patterns: cytoplasmic (cANCA), most often linked to anti-PR3, perinuclear (pANCA), most often linked to anti-MPO, and patterns unrelated to AAV. Incorporating formalin-fixed granulocytes into the workflow enhances pattern differentiation, helping to distinguish true ANCA from patterns unrelated to AAV, i.g. interfering ANA, thereby increasing diagnostic specificity (see figure below).
In clinical practice, ANCA testing is often performed sequentially: initial screening with ethanol-fixed granulocytes, followed by formalin-fixed granulocyte IFA, and ultimately confirmed with antigen-specific assays. Or in some cases, it relies on a single IFA ANCA test alone. While effective, those approaches can delay results or create uncertainty in interpretation, particularly in patients with overlapping clinical features.
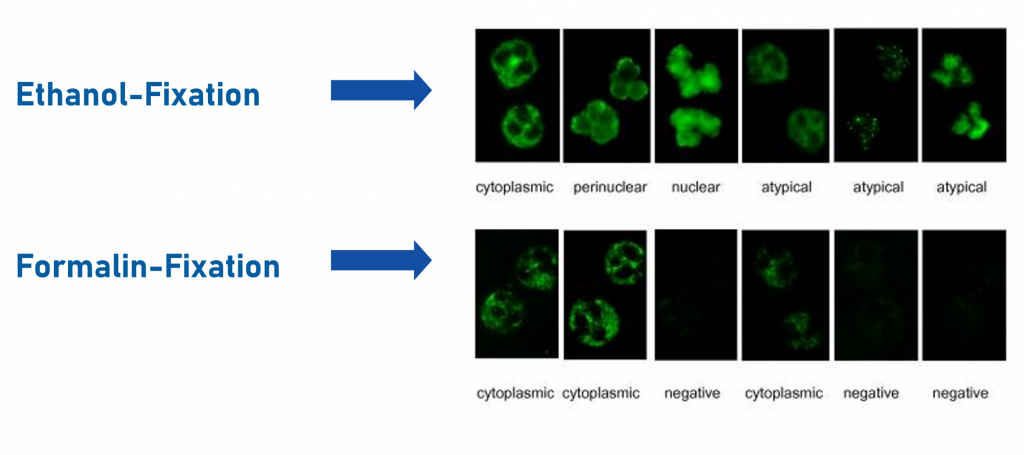
Different pattern on ethanol-fixed and formalin-fixed granulocytes, adapted from Knütter, et al. (2012) 1
Introducing the ANCA Dual Test
To address these challenges, Medipan & GA Generic Assays have developed the ANCA Dual Test — an innovative IFA-based solution that allows testing the same patient sample simultaneously on ethanol-fixed and formalin-fixed granulocytes using a single slide. This dual-substrate approach enables the simultaneous detection and differentiation of cANCA and pANCA patterns, improving diagnostic precision and reliability.
Key Advantages
- Two substrates, one slide: Ethanol- and formalin-fixed granulocytes tested in parallel on the same slide.
- Improved pattern recognition: Easier distinction between cANCA, pANCA, and other patterns unrelated to AAV
- Time-saving and workflow-friendly: Reduces turnaround time and lab workload compared to sequential substrate testing.
- High clinical relevance: Supports classification and disease monitoring
- Automation-ready: Compatible with the IFA reading system akiron®NEO and with standard slide processing platforms, i.e. AP 16 IF ELITE.
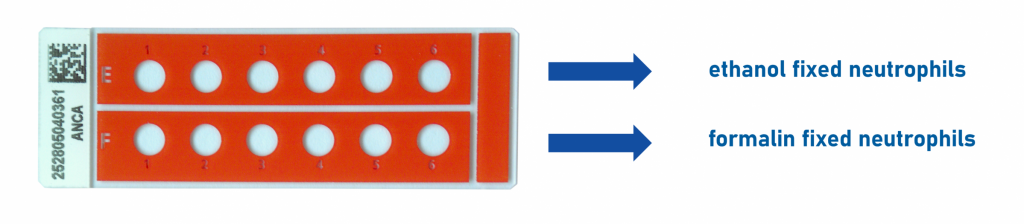
ANCA dual slide
Why It Matters Now
Recent updates to international guidelines emphasize the role of ANCA serology in disease definition and diagnosis. The 2022 ACR/EULAR classification criteria for GPA2 and MPA3 assign the highest point values to cANCA and pANCA positivity whereas the 2020 consensus on ANCA testing beyond systemic vasculitis4 highlights the clinical and diagnostic value of ANCA testing in patients with various autoimmune, infectious and neoplastic diseases. The ability to evaluate both substrate reactions simultaneously provides a more accurate serological profile and facilitates earlier clinical decisions, particularly in nuclear, atypical, or overlapping cases.
Clinical Applications
- Initial diagnostic screening of suspected AAV patients and in certain settings beyond systemic vasculitis
- Reflex testing following unclear antigen-specific immunoassays for anti-PR3 and -MPO
- Confirmation and classification of GPA vs MPA
- Monitoring of ANCA levels in disease follow-up and relapse detection
Get Started with ANCA Dual
For laboratories seeking to streamline autoimmune vasculitis diagnostics, it represents a practical and clinically meaningful upgrade.
✉️ Contact us to request the product IFU or an evaluation kit.
Related products
| IFA for standard microscope | 87261 – ANCA IFA dual | Determination of IgG antibodies against neutrophil cytoplasmic antigens (ANCA), using slides coated with ethanol- and formalin-fixed human granulocytes. |
| IFA for akiron®NEO | 4472 – AKLIDES® ANCA dual |
Discover the full range of the ANCA IFA Assays
References
- Knütter, et al. (2012), Automated interpretation of ANCA patterns – a new approach in the serology of ANCA-associated vasculitis ↩︎
- Robson, et al. (2022), 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for granulomatosis with polyangiitis (GPA) ↩︎
- Suppiah, et al. (2022), 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for microscopic polyangiitis (MPA) ↩︎
- Moiseev, et al. (2020) ,2020 international consensus on ANCA testing beyond
systemic vasculitis ↩︎