8065 – CytoBead® ANA

Highlights
- Qualitative and semi-quantitative detection of IgG antibodies against nuclear and cytoplasmic antigens (dsDNA, Scl-70, SS-A/Ro60, SS-A/Ro52, SS-B, CENP-B, Sm, Sm/RNP)
- Supports the diagnosis of systemic autoimmune diseases
- Ready-to-use reagents for manual processing
- Quality assured handling in routine laboratories
- Consistent processing for parallel use of multiple IF assays
- High diagnostic sensitivity and specificity
- Automated imaging using AKLIDES® or akiron®NEO systems
- CE marked
Intended Use
The CytoBead® ANA is an immunofluorescence assay (IFA) for the qualitative and semi-quantitative determination IgG antibodies against nuclear and cytoplasmic antigens (dsDNA, Scl-70, SS-A/Ro60, SS-A/Ro52, SS-B, Sm, Sm/RNP and CENP-B) in human serum. The CytoBead® ANA is is intended as an aid in the diagnosis of systemic autoimmune diseases in conjunction with other clinical and laboratory findings. The immunoassay is designed for manual professional in vitro diagnostic use.
Diagnostic Relevance
Autoantibodies targeting components of the cell nucleus are hallmark features of systemic autoimmune diseases, including systemic lupus erythematosus (SLE), Sjögren’s syndrome, progressive systemic sclerosis (PSS), mixed connective tissue disease (MCTD), rheumatoid arthritis (RA), and dermatomyositis. Detection of these antibodies in patient serum or plasma is a well-established practice that provides critical diagnostic information and can guide disease management.
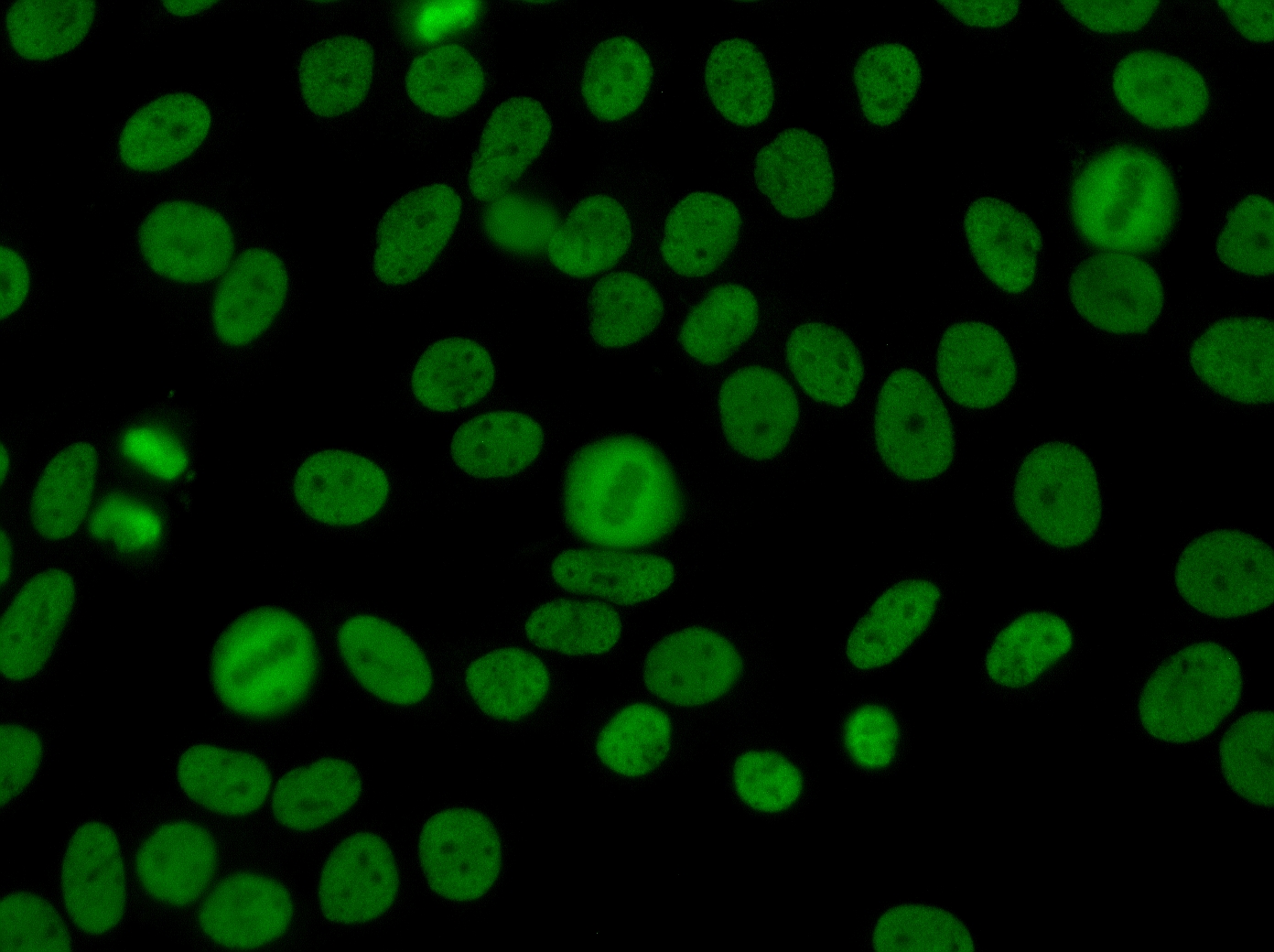
The use of HEp-2 cells in immunofluorescence assays offers a highly sensitive method for detecting antinuclear antibodies (ANA). Staining patterns observed on HEp-2 cells allow clinicians to identify the specific target antigens of the ANA, which can indicate the underlying autoimmune disorder and inform clinical decision-making. By combining qualitative and semi-quantitative analysis, the CytoBead® ANA not only confirms the presence of autoantibodies but also provides insight into their relative concentration, helping monitor disease progression, assess treatment response, and support early intervention in patients with systemic autoimmune diseases.
Publications
- Zaripova et al., (2025) SSc with Interstitial Lung Disease-Identification of Novel Immunogenetic Markers and Ethnic Specificity in Kazakh Patients
- Fitriah et al., (2023) The Discrepancy of ANA and Compartment Bead Patterns Suggestive of a Neuropsychiatry SLE
- Fitriah et al., (2017) Next-Generation Autoantibody Testing by Combination of Screening and Confirmation—the CytoBead® Technology
- Sowa et al., (2014) Der CytoBead-Assay – Eine neue Möglichkeit der multiparametrischen AutoAbsanalytik bei systemischen Autoimmunerkrankungen
Product Specifications
| Title | CytoBead® ANA |
| Product code | 8065 |
| Indication | Systemic autoimmune diseases |
| Description | Indirect immunofluorescence assay for the determination of IgG antibodies in human serum against nuclear and cytoplasmic antigens |
| Format | Slides coated with HEp-2 cells as well as with beads coated with the ANA antigens dsDNA, Scl-70, SS-A/Ro60, SS-A/Ro52, SS-B, Sm, Sm/RNP, and CENP-B |
| Total incubation time | 60 min. |
| Sample volume | 5 µL serum |
| No. of determinations | 80 (10 x 8) |
Free downloads
SDS [REF 8065][eng] SDS [REF 8065][deu] Flyer [CytoBead Technology][eng] Flyer [IFA Product Portfolio][eng]Restricted downloads - Password required
Current version of the instructions for use. The respective valid version for processing the test can be found in the product packaging.